
Составьте уравнения реакций к схеме 14.
Схема 14
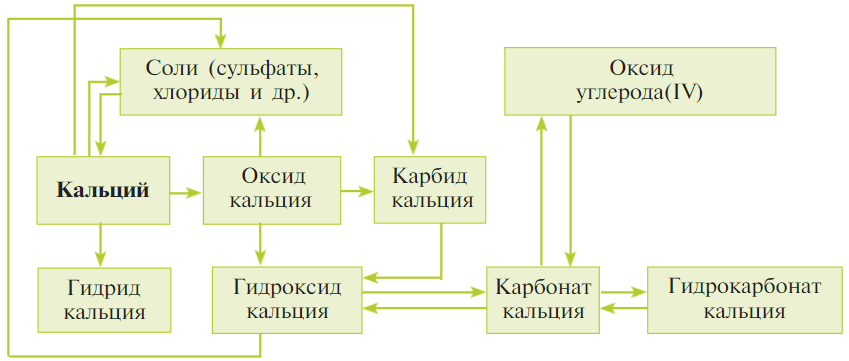

1) $Ca + H_{2} = CaH_{2}$
2) $Ca + 2HCl = CaCl_{2} + H_{2}$
3) $2Ca + O_{2} = 2CaO$
4) $Ca + 2C = CaC_{2}$
5) $CaO + 2HCl = CaCl_{2} + H_{2}O$
6) $CaO + 3C = CaC_{2} + CO$
7) $CaO + H_{2}O = Ca(OH)_{2}$
8) $CaC_{2} + 2H_{2}O = Ca(OH)_{2} + C_{2}H_{2}$
9) $Ca(OH)_{2} + 2HCl = CaCl_{2} + 2H_{2}O$
10) $Ca(OH)_{2} + CO_{2} = CaCO_{3} ↓ + 2H_{2}O$
11) $CaCO_{3} = CaO + CO_{2}↑$
12) $CaCO_{3} + CO_{2} + H_{2}O = Ca(HCO_{3})_{2}$
13) $Ca(HCO_{3})_{2} = CaCO_{3}↓ + CO_{2}↑ + H_{2}O$
Пожалуйста, оцените решение